Opioid
(Updated October 2021)
Key messages:
- The overall rate of both strong and weak opioid use has not increased since the previous report. However, in those aged 80 and over the rate continues to rise.
- Rates of opioid dispensing is higher in people of European/Other ethnicity, women and people aged 80 and over.
- Almost half of those dispensed a strong opioid had a ‘trigger event’ in a public hospital in the week prior, suggesting these prescriptions are generated in hospital.
- Rates of fentanyl use have more than doubled since 2011 and the variation has increased to more than 15-fold.
The rate of strong opioid dispensing has decreased since 2016
- A ‘strong’ opioid is one classed as step 3 of the World Health Organization (WHO) analgesic ladder. In Aotearoa New Zealand the following strong opioids are subsidised: fentanyl, methadone, morphine, oxycodone and pethidine.
- Excluding people receiving methadone for opioid substitution treatment, in 2019, an average of 16.6 per 1,000 people in Aotearoa New Zealand received a strong opioid. This varied more than two-fold between DHBs.
- People identifying as European/Other ethnicity were dispensed 2–5 times as many strong opioids as those from other ethnic groups.
- Rates for Asian and Pacific peoples have remained constant since 2015.
- Use increased significantly with each age group; on average, 11 percent of people aged 80 years and over were dispensed a strong opioid in 2017.
- Women were dispensed significantly more strong opioids than men.
- Of the people receiving a strong opioid, 12 percent took the opioid for six or more weeks. People aged 65 years and over were at least three times more likely to be dispensed a strong opioid for six or more weeks. Rates have decreased since 2015.
Table 1: Rate per 1,000 dispensing of strong opioid medicines, by age and ethnicity, 2019
| Age (years) | Māori | Pacific peoples | Asian | European/Other | Total |
| 0–24 | 1.3 | 1.2 | 0.5 | 2.7 | 1.9 |
| 25–64 | 16.5 | 10.0 | 3.6 | 15.9 | 13.5 |
| 65–79 | 45.3 | 36.4 | 16.2 | 38.6 | 37.5 |
| 80 and over | 88.1 | 82.8 | 59.4 | 117.2 | 112.5 |
| Total | 11.3 | 8.1 | 3.9 | 21.5 | 16.2 |
Weak opioid use increases with age
- A ‘weak’ opioid is classed as step 2 of the WHO analgesic ladder. In Aotearoa New Zealand the following weak opioids are subsidised: tramadol, codeine and dihydrocodeine. Paracetamol with codeine was excluded because it contains a comparatively low dose of codeine.
- In 2019, an average of 98.8 per 1,000 people in Aotearoa New Zealand received a weak opioid. This rate has been decreasing consistently since 2015, when the rate was around 104 per 1,000. This decrease occurs across all age groups, but not for Māori and Pacific peoples.
- At all ages, people identifying as European/Other received over twice as many weak opioids compared with Asian people.
- Use increased significantly with age; on average 18 percent of people aged 80 years and over received a weak opioid in 2019.
- Nationally, tramadol was dispensed to fewer people than codeine or dihydrocodeine – 46.9 per 1,000 compared with 62 per 1,000.
- Tramadol use in people aged 80 years and over has been decreasing steadily from 70 per 1,000 people in 2013 to 49 per 1,000 in 2019.
- As with strong opioids, women were dispensed significantly more weak opioids than men.
Morphine use has increased since 2011
- In 2019, an average of 11 per 1,000 people received morphine. Use varied three-fold between DHBs, an increase in variation from the previous report.
- Of those dispensed a strong opioid in 2019, two-thirds received morphine.
- The number of people dispensed morphine has increased by 21,000 people since 2011, from 7.5 to 11.0 per 1,000 people in 2019.
- Of every 10 people given morphine, 1.1 took it for six or more weeks and 9.3 among people who were European/Other aged 80 and over.
Table 2: Rate per 1,000 dispensing of morphine by age, 2011–19
| Age (years) | 2011 | 2017 | 2018 | 2019 | Rate increase | |
| 0–24 | 0.8 | 1.6 | 1.5 | 1.4 | 0.6 | |
| 25–64 | 6.1 | 9.7 | 9.5 | 9.4 | 3.3 | |
| 65–79 | 20.6 | 25.9 | 25.2 | 25.1 | 4.5 | |
| 80 and over | 59.6 | 75.6 | 73.0 | 73.4 | 13.8 | |
| Total | 7.5 | 11.2 | 10.9 | 11.0 | 3.5 | |
| Count people | 32,883 | 53,624 | 52,871 | 54,644 |
Oxycodone use has remained the same since 2016
- In 2019, an average of 5.3 per 1,000 people received oxycodone.
- The number of people dispensed oxycodone has significantly decreased by 27 percent since 2011, from 7.4 to 5.4 per 1,000 people in 2019.
- Use varied over nine-fold between DHBs.
- Of every 10 people given oxycodone, 1.2 took it for six or more weeks.
Rates of fentanyl use vary widely, particularly in those aged 80 and over
- In 2019, an average of 1.6 per 1,000 people received fentanyl. This was a significant increase from 2011 (0.8 per 1,000), however the rate has decreased since 2016. Use varied over 12-fold between DHBs.
- Fentanyl use significantly increased with age from 4.0 per 1,000 aged 65–79 years to 20.3 per 1,000 people aged 80 years and over.
- Of those given fentanyl, 20 percent took it for six or more weeks.
Almost 40 percent of those dispensed a strong opioid had a public hospital ‘trigger event’
- Of every 10 people dispensed a strong opioid, nearly half attended a public hospital as an inpatient or outpatient in the week prior.
- Younger people were more likely to have a public hospital event prior to being dispensed a strong opioid compared with older people; 7 out of every 10 of those aged 0–24 years compared with 4 out of 10 of those aged 80 years and over.
In previous Atlases we have noticed an increasingly high rate of morphine use in people aged 65 and over living in aged residential care (ARC). In contrast, the rate of morphine use by people aged 65 and over not living in ARC is not increasing at the same rate.
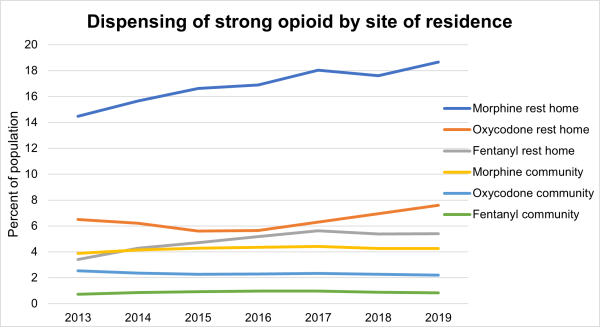
We undertook further investigation to explore what is causing the increase in ARC.
The ARC population was defined as those listed in the ARC funding database at any point in the year of interest. The non-ARC population may include people receiving palliative care in hospital or hospices (excluding facilities in ARC). The data below does not include medicines dispensed in hospital. Please note we are measuring medicine that is dispensed but not necessarily taken.
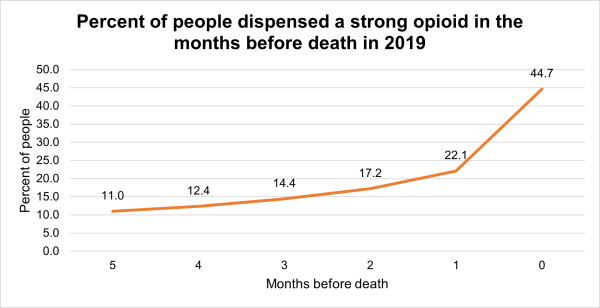
One of the most likely reasons for the difference in strong opioid use between community and ARC populations could be the use of strong opioids for palliative care. Morphine is the recommended first-line opioid in palliative care.[1] The dispensing of strong opioids by type in the six months prior to death was analysed. The graph below shows strong opioid dispensing rates were highest in the month in which people die, with 54 percent of ARC residents and 40 percent of non-ARC residents dispensed a strong opioid in their last month of life. Overall, in 2019, 61 percent of those in ARC and 45 percent of those in non-ARC were dispensed a strong opioid at some point in their last six of months of life.
The table below provides a summary:
|
People aged 65 years and over dispensed a strong opioid, percent |
2017 |
2018 |
2019 |
|
All people 65 years and over |
5.8 |
5.6 |
5.5 |
|
People in their last 6 months of life |
49.7 |
50.5 |
50.6 |
Conclusion: Much of the differences in strong opioid dispensing observed between people in aged residential care and those who were not can be explained by strong opioid dispensing to people in their last six months of life.
Questions this analysis might prompt:
- What are the indications for strong opioid dispensing? Are strong opioids being dispensed in accordance with best-practice evidence?
- How many people are being given strong opioids for longer than six weeks?
The Institute for Healthcare Improvement (IHI) classes opioids as one of four groups of medicines (along with anticoagulants, insulin and sedatives) that can cause harm to patients, even when used as intended.
Opioid analgesia is the primary intervention for managing pain in hospital patients. Opioids are also considered effective treatment for severe pain in palliative care.[2] In the non-specialist setting, the National Institute for Health and Care Excellence (NICE)[3] recommends opioids should not be used for neuropathic pain without specialist assessment. A 2013 review noted increases in both opioid use for back pain and other chronic musculoskeletal pain conditions, and in prescription opioid addiction and fatal overdose.[4]
Despite the increasing use of chronic opioid therapy, there is limited evidence that opioids are effective for treating chronic non-cancer pain in the long term. A 2013 Cochrane review concluded that long-term use of opioids should be undertaken with extreme caution and should consider the potential for serious adverse effects and complications.[5] Harms associated with opioid therapy include opioid tolerance, opioid-induced hyperalgesia,[6] iatrogenic addiction and dependency, drug diversion and aberrant drug-related behaviours.[7] In addition, a strong relationship between severe dependence on opioids and other substance use and mental health disorders has been observed.[8]
It is recommended that health practitioners considering prescribing opioids for chronic non-malignant pain carefully undertake a benefit-to-harm evaluation to ensure opioid therapy is the best option. The evaluation should include taking a history, undertaking a physical examination and performing a full diagnosis before starting opioid therapy. Evaluation should be ongoing for the duration of the therapy.[9]
Data for this Atlas domain was drawn from the Pharmaceutical Collection, which contains claim and payment information from community pharmacists for subsidised dispensing. This collection does not allow for analysis of patients’ condition or the effectiveness of dose provided. This means it was not possible to assess the appropriateness or otherwise of prescribing. Unsubsidised dispensing is not included in this analysis; nor does it indicate if people took the medicine.
- The methodology is provided here (256KB, PDF).
Note this analysis focuses on the number of people dispensed opioids, not on the number of prescriptions of these medicines. We have used this approach because interpreting what the number of dispensed prescriptions might mean is complicated by differences in prescribing frequency, formulation and dose, and if the medicine is to be taken ‘as needed’ or at regular times. We recommend further local analysis on the people receiving these medicines that accounts for these factors and the person’s clinical condition.
What questions might the data prompt?
- Why do some DHBs have consistently lower or higher rates than the national mean?
- How do DHBs with similar populations compare?
- What are your DHB’s trends for opioid prescribing in aged residential care?
- What is the effect of a DHB’s access to specialist pain services on the use of opioids?
- What is the effect of access to palliative care services?
- What impact might access to non-pharmacologic pain management have on these rates?
- What tools and skills do primary care providers have to manage chronic non-cancer pain?
- Why are there marked ethnic differences in the use of opioids? Is it the result of higher use in older people or might it reflect other differences, such as different cultural expressions of pain and different ways of coping with pain?
- What other combinations of medicines are people receiving strong opioids for six or more weeks also receiving? Additional analysis showed 4 in every 10 of those on strong opioids received a benzodiazepine in the same period.
- What is the extent of polypharmacy in older people who are using opioids?
- How does the falls rate of older people in your DHB who are using opioids compare with the rate of those who are not?
- What regular pain management medicines are used by older people who have had a fall?
- bpacnz. 2014. Identifying and managing addiction to opioids. Best Practice Journal 64. URL: www.bpac.org.nz/BPJ/2014/October/opioid-addiction.aspx.
- bpacnz. 2014. Helping patients cope with chronic non-malignant pain: it’s not about opioids. Best Practice Journal 63. URL: www.bpac.org.nz/BPJ/2014/September/chronicpain.aspx.
- bpacnz. 2014. Upfront: “A disaster in the making”: it’s time to take action against misuse of oxycodone. Best Practice Journal 61. URL: www.bpac.org.nz/BPJ/2014/June/upfront.aspx.
- bpacnz. 2012. Strong opioids for pain management in adults in palliative care. Best Practice Journal 49. URL: www.bpac.org.nz/bpj/2012/december/opioids.aspx.
- bpacnz. 2016. Oxycodone update. bpacnzreports. URL: www.bpac.org.nz/Report/2016/February/oxycodone.aspx.
- The Royal Australasian College of Physicians. 2009. Prescription Opioid Policy: Improving management of chronic non-malignant pain and prevention of problems associated with prescription opioid use. Sydney: The Royal Australasian College of Physicians. URL: https://www.ranzcp.org/clinical-guidelines-publications/clinical-guidelines-publications-library/opiod-policy.
- Roxburgh A, Bruno R, Larance B, et al. 2011. Prescription of opioid analgesics and related harms in Australia. Med J Aust 195(5): 280–4. URL: www.mja.com.au/journal/2011/195/5/prescription-opioid-analgesics-and-related-harms-australia.
- Scottish Intercollegiate Guidelines Network (SIGN). 2013. Management of chronic pain. Edinburgh: SIGN. (SIGN publication no. 136). [December 2013]. URL: www.sign.ac.uk.
- Ministry of Health. 2017. Te Ara Whakapiri Toolkit: Care in the last days of life. Wellington: Ministry of Health. URL: www.health.govt.nz/system/files/documents/publications/te-ara-whakapiri-toolkit-apr17.docx.
- bpacnz. 2012. Strong opioids for pain management in adults in palliative care. Best Practice Journal 49. URL: www.bpac.org.nz/bpj/2012/december/docs/bpj_49_opioids_pages_8-17.pdf.
- NICE. 2013. Do not use morphine to treat neuropathic pain in non-specialist settings, unless advised by a specialist to do so. URL: www.nice.org.uk/donotdo/do-not-use-morphine-to-treat-neuropathic-pain-in-nonspecialist-settingsunless-advised-by-a-specialist-to-do-so (accessed 20 June 2016).
- Korff M. 2013. Long-term Use of Opioids for Complex Chronic Pain. Best Pract Res Clin Rheumatol 27(5): 663–72.
- Chaparro LE, Furlan AD, Deshpande A, et al. Opioids compared to placebo or other treatments for chronic low-back pain. Cochrane Database Syst Rev 2013, Issue 8. Art. No.: CD004959. DOI: 10.1002/14651858.CD004959.pub4.
- Chen L, Vo T, Seefeld L, et al. 2013. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain 14(4): 384–92.
- Sullivan MD, Von Korff M, Banta-Green C, et al. 2010. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain 149(2): 345–53.
- The Royal Australasian College of Physicians. 2009. Prescription Opioid Policy: Improving management of chronic non-malignant pain and prevention of problems associated with prescription opioid use. Sydney: The Royal Australasian College of Physicians.
- Chou R, Fanciullo GJ, Fine PG, et al. 2009. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 10(2): 113–30. DOI: 10.1016/j.jpain.2008.10.00.
Update with 2019 data
(Updated October 2021)
Key messages:
- The overall rate of both strong and weak opioid use has not increased since the previous report. However, in those aged 80 and over the rate continues to rise.
- Rates of opioid dispensing is higher in people of European/Other ethnicity, women and people aged 80 and over.
- Almost half of those dispensed a strong opioid had a ‘trigger event’ in a public hospital in the week prior, suggesting these prescriptions are generated in hospital.
- Rates of fentanyl use have more than doubled since 2011 and the variation has increased to more than 15-fold.
Key findings
The rate of strong opioid dispensing has decreased since 2016
- A ‘strong’ opioid is one classed as step 3 of the World Health Organization (WHO) analgesic ladder. In Aotearoa New Zealand the following strong opioids are subsidised: fentanyl, methadone, morphine, oxycodone and pethidine.
- Excluding people receiving methadone for opioid substitution treatment, in 2019, an average of 16.6 per 1,000 people in Aotearoa New Zealand received a strong opioid. This varied more than two-fold between DHBs.
- People identifying as European/Other ethnicity were dispensed 2–5 times as many strong opioids as those from other ethnic groups.
- Rates for Asian and Pacific peoples have remained constant since 2015.
- Use increased significantly with each age group; on average, 11 percent of people aged 80 years and over were dispensed a strong opioid in 2017.
- Women were dispensed significantly more strong opioids than men.
- Of the people receiving a strong opioid, 12 percent took the opioid for six or more weeks. People aged 65 years and over were at least three times more likely to be dispensed a strong opioid for six or more weeks. Rates have decreased since 2015.
Table 1: Rate per 1,000 dispensing of strong opioid medicines, by age and ethnicity, 2019
| Age (years) | Māori | Pacific peoples | Asian | European/Other | Total |
| 0–24 | 1.3 | 1.2 | 0.5 | 2.7 | 1.9 |
| 25–64 | 16.5 | 10.0 | 3.6 | 15.9 | 13.5 |
| 65–79 | 45.3 | 36.4 | 16.2 | 38.6 | 37.5 |
| 80 and over | 88.1 | 82.8 | 59.4 | 117.2 | 112.5 |
| Total | 11.3 | 8.1 | 3.9 | 21.5 | 16.2 |
Weak opioid use increases with age
- A ‘weak’ opioid is classed as step 2 of the WHO analgesic ladder. In Aotearoa New Zealand the following weak opioids are subsidised: tramadol, codeine and dihydrocodeine. Paracetamol with codeine was excluded because it contains a comparatively low dose of codeine.
- In 2019, an average of 98.8 per 1,000 people in Aotearoa New Zealand received a weak opioid. This rate has been decreasing consistently since 2015, when the rate was around 104 per 1,000. This decrease occurs across all age groups, but not for Māori and Pacific peoples.
- At all ages, people identifying as European/Other received over twice as many weak opioids compared with Asian people.
- Use increased significantly with age; on average 18 percent of people aged 80 years and over received a weak opioid in 2019.
- Nationally, tramadol was dispensed to fewer people than codeine or dihydrocodeine – 46.9 per 1,000 compared with 62 per 1,000.
- Tramadol use in people aged 80 years and over has been decreasing steadily from 70 per 1,000 people in 2013 to 49 per 1,000 in 2019.
- As with strong opioids, women were dispensed significantly more weak opioids than men.
Morphine use has increased since 2011
- In 2019, an average of 11 per 1,000 people received morphine. Use varied three-fold between DHBs, an increase in variation from the previous report.
- Of those dispensed a strong opioid in 2019, two-thirds received morphine.
- The number of people dispensed morphine has increased by 21,000 people since 2011, from 7.5 to 11.0 per 1,000 people in 2019.
- Of every 10 people given morphine, 1.1 took it for six or more weeks and 9.3 among people who were European/Other aged 80 and over.
Table 2: Rate per 1,000 dispensing of morphine by age, 2011–19
| Age (years) | 2011 | 2017 | 2018 | 2019 | Rate increase | |
| 0–24 | 0.8 | 1.6 | 1.5 | 1.4 | 0.6 | |
| 25–64 | 6.1 | 9.7 | 9.5 | 9.4 | 3.3 | |
| 65–79 | 20.6 | 25.9 | 25.2 | 25.1 | 4.5 | |
| 80 and over | 59.6 | 75.6 | 73.0 | 73.4 | 13.8 | |
| Total | 7.5 | 11.2 | 10.9 | 11.0 | 3.5 | |
| Count people | 32,883 | 53,624 | 52,871 | 54,644 |
Oxycodone use has remained the same since 2016
- In 2019, an average of 5.3 per 1,000 people received oxycodone.
- The number of people dispensed oxycodone has significantly decreased by 27 percent since 2011, from 7.4 to 5.4 per 1,000 people in 2019.
- Use varied over nine-fold between DHBs.
- Of every 10 people given oxycodone, 1.2 took it for six or more weeks.
Rates of fentanyl use vary widely, particularly in those aged 80 and over
- In 2019, an average of 1.6 per 1,000 people received fentanyl. This was a significant increase from 2011 (0.8 per 1,000), however the rate has decreased since 2016. Use varied over 12-fold between DHBs.
- Fentanyl use significantly increased with age from 4.0 per 1,000 aged 65–79 years to 20.3 per 1,000 people aged 80 years and over.
- Of those given fentanyl, 20 percent took it for six or more weeks.
Almost 40 percent of those dispensed a strong opioid had a public hospital ‘trigger event’
- Of every 10 people dispensed a strong opioid, nearly half attended a public hospital as an inpatient or outpatient in the week prior.
- Younger people were more likely to have a public hospital event prior to being dispensed a strong opioid compared with older people; 7 out of every 10 of those aged 0–24 years compared with 4 out of 10 of those aged 80 years and over.
Use of medication by site of residence | 65 and over (2021)
In previous Atlases we have noticed an increasingly high rate of morphine use in people aged 65 and over living in aged residential care (ARC). In contrast, the rate of morphine use by people aged 65 and over not living in ARC is not increasing at the same rate.

We undertook further investigation to explore what is causing the increase in ARC.
The ARC population was defined as those listed in the ARC funding database at any point in the year of interest. The non-ARC population may include people receiving palliative care in hospital or hospices (excluding facilities in ARC). The data below does not include medicines dispensed in hospital. Please note we are measuring medicine that is dispensed but not necessarily taken.
One of the most likely reasons for the difference in strong opioid use between community and ARC populations could be the use of strong opioids for palliative care. Morphine is the recommended first-line opioid in palliative care.[1] The dispensing of strong opioids by type in the six months prior to death was analysed. The graph below shows strong opioid dispensing rates were highest in the month in which people die, with 54 percent of ARC residents and 40 percent of non-ARC residents dispensed a strong opioid in their last month of life. Overall, in 2019, 61 percent of those in ARC and 45 percent of those in non-ARC were dispensed a strong opioid at some point in their last six of months of life.

The table below provides a summary:
|
People aged 65 years and over dispensed a strong opioid, percent |
2017 |
2018 |
2019 |
|
All people 65 years and over |
5.8 |
5.6 |
5.5 |
|
People in their last 6 months of life |
49.7 |
50.5 |
50.6 |
Conclusion: Much of the differences in strong opioid dispensing observed between people in aged residential care and those who were not can be explained by strong opioid dispensing to people in their last six months of life.
Questions this analysis might prompt:
- What are the indications for strong opioid dispensing? Are strong opioids being dispensed in accordance with best-practice evidence?
- How many people are being given strong opioids for longer than six weeks?
Background
The Institute for Healthcare Improvement (IHI) classes opioids as one of four groups of medicines (along with anticoagulants, insulin and sedatives) that can cause harm to patients, even when used as intended.
Opioid analgesia is the primary intervention for managing pain in hospital patients. Opioids are also considered effective treatment for severe pain in palliative care.[2] In the non-specialist setting, the National Institute for Health and Care Excellence (NICE)[3] recommends opioids should not be used for neuropathic pain without specialist assessment. A 2013 review noted increases in both opioid use for back pain and other chronic musculoskeletal pain conditions, and in prescription opioid addiction and fatal overdose.[4]
Despite the increasing use of chronic opioid therapy, there is limited evidence that opioids are effective for treating chronic non-cancer pain in the long term. A 2013 Cochrane review concluded that long-term use of opioids should be undertaken with extreme caution and should consider the potential for serious adverse effects and complications.[5] Harms associated with opioid therapy include opioid tolerance, opioid-induced hyperalgesia,[6] iatrogenic addiction and dependency, drug diversion and aberrant drug-related behaviours.[7] In addition, a strong relationship between severe dependence on opioids and other substance use and mental health disorders has been observed.[8]
It is recommended that health practitioners considering prescribing opioids for chronic non-malignant pain carefully undertake a benefit-to-harm evaluation to ensure opioid therapy is the best option. The evaluation should include taking a history, undertaking a physical examination and performing a full diagnosis before starting opioid therapy. Evaluation should be ongoing for the duration of the therapy.[9]
Data sources and method
Data for this Atlas domain was drawn from the Pharmaceutical Collection, which contains claim and payment information from community pharmacists for subsidised dispensing. This collection does not allow for analysis of patients’ condition or the effectiveness of dose provided. This means it was not possible to assess the appropriateness or otherwise of prescribing. Unsubsidised dispensing is not included in this analysis; nor does it indicate if people took the medicine.
- The methodology is provided here (256KB, PDF).
Note this analysis focuses on the number of people dispensed opioids, not on the number of prescriptions of these medicines. We have used this approach because interpreting what the number of dispensed prescriptions might mean is complicated by differences in prescribing frequency, formulation and dose, and if the medicine is to be taken ‘as needed’ or at regular times. We recommend further local analysis on the people receiving these medicines that accounts for these factors and the person’s clinical condition.
What questions might the data prompt?
- Why do some DHBs have consistently lower or higher rates than the national mean?
- How do DHBs with similar populations compare?
- What are your DHB’s trends for opioid prescribing in aged residential care?
- What is the effect of a DHB’s access to specialist pain services on the use of opioids?
- What is the effect of access to palliative care services?
- What impact might access to non-pharmacologic pain management have on these rates?
- What tools and skills do primary care providers have to manage chronic non-cancer pain?
- Why are there marked ethnic differences in the use of opioids? Is it the result of higher use in older people or might it reflect other differences, such as different cultural expressions of pain and different ways of coping with pain?
- What other combinations of medicines are people receiving strong opioids for six or more weeks also receiving? Additional analysis showed 4 in every 10 of those on strong opioids received a benzodiazepine in the same period.
- What is the extent of polypharmacy in older people who are using opioids?
- How does the falls rate of older people in your DHB who are using opioids compare with the rate of those who are not?
- What regular pain management medicines are used by older people who have had a fall?
Recommended reading
- bpacnz. 2014. Identifying and managing addiction to opioids. Best Practice Journal 64. URL: www.bpac.org.nz/BPJ/2014/October/opioid-addiction.aspx.
- bpacnz. 2014. Helping patients cope with chronic non-malignant pain: it’s not about opioids. Best Practice Journal 63. URL: www.bpac.org.nz/BPJ/2014/September/chronicpain.aspx.
- bpacnz. 2014. Upfront: “A disaster in the making”: it’s time to take action against misuse of oxycodone. Best Practice Journal 61. URL: www.bpac.org.nz/BPJ/2014/June/upfront.aspx.
- bpacnz. 2012. Strong opioids for pain management in adults in palliative care. Best Practice Journal 49. URL: www.bpac.org.nz/bpj/2012/december/opioids.aspx.
- bpacnz. 2016. Oxycodone update. bpacnzreports. URL: www.bpac.org.nz/Report/2016/February/oxycodone.aspx.
- The Royal Australasian College of Physicians. 2009. Prescription Opioid Policy: Improving management of chronic non-malignant pain and prevention of problems associated with prescription opioid use. Sydney: The Royal Australasian College of Physicians. URL: https://www.ranzcp.org/clinical-guidelines-publications/clinical-guidelines-publications-library/opiod-policy.
- Roxburgh A, Bruno R, Larance B, et al. 2011. Prescription of opioid analgesics and related harms in Australia. Med J Aust 195(5): 280–4. URL: www.mja.com.au/journal/2011/195/5/prescription-opioid-analgesics-and-related-harms-australia.
- Scottish Intercollegiate Guidelines Network (SIGN). 2013. Management of chronic pain. Edinburgh: SIGN. (SIGN publication no. 136). [December 2013]. URL: www.sign.ac.uk.
References
- Ministry of Health. 2017. Te Ara Whakapiri Toolkit: Care in the last days of life. Wellington: Ministry of Health. URL: www.health.govt.nz/system/files/documents/publications/te-ara-whakapiri-toolkit-apr17.docx.
- bpacnz. 2012. Strong opioids for pain management in adults in palliative care. Best Practice Journal 49. URL: www.bpac.org.nz/bpj/2012/december/docs/bpj_49_opioids_pages_8-17.pdf.
- NICE. 2013. Do not use morphine to treat neuropathic pain in non-specialist settings, unless advised by a specialist to do so. URL: www.nice.org.uk/donotdo/do-not-use-morphine-to-treat-neuropathic-pain-in-nonspecialist-settingsunless-advised-by-a-specialist-to-do-so (accessed 20 June 2016).
- Korff M. 2013. Long-term Use of Opioids for Complex Chronic Pain. Best Pract Res Clin Rheumatol 27(5): 663–72.
- Chaparro LE, Furlan AD, Deshpande A, et al. Opioids compared to placebo or other treatments for chronic low-back pain. Cochrane Database Syst Rev 2013, Issue 8. Art. No.: CD004959. DOI: 10.1002/14651858.CD004959.pub4.
- Chen L, Vo T, Seefeld L, et al. 2013. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain 14(4): 384–92.
- Sullivan MD, Von Korff M, Banta-Green C, et al. 2010. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain 149(2): 345–53.
- The Royal Australasian College of Physicians. 2009. Prescription Opioid Policy: Improving management of chronic non-malignant pain and prevention of problems associated with prescription opioid use. Sydney: The Royal Australasian College of Physicians.
- Chou R, Fanciullo GJ, Fine PG, et al. 2009. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 10(2): 113–30. DOI: 10.1016/j.jpain.2008.10.00.
