Community use of antibiotics
Antimicrobial resistance is emerging as a problem worldwide. Overuse of antibiotics is one of the causes of antimicrobial resistance.
In Aotearoa New Zealand, up to 95 percent of antibiotics are dispensed in the community[1], suggesting that a focus on promoting appropriate community use is an important way to address antimicrobial resistance. Recent analysis indicates there is likely to be a mix of underuse and overuse of antibacterials relative to health need, particularly in Māori and Pacific peoples. This suggests the balance of under- and over-prescribing of antibiotics in Aotearoa New Zealand needs to be better understood[2].
The purpose of this Atlas of Healthcare Variation domain is to highlight regional and demographic variation in community antibiotic use, with the goal of prompting debate and raising questions about why differences exist. Professor John Wennberg defined unwarranted variation as being variation that cannot be explained by differences in either patient need or patient preference. This Atlas domain invites readers to ask questions to understand how much antibiotic prescribing can be attributed to patient need and how much might reflect unwarranted variation[3].
Data comes from the Pharmaceutical Collection and there are limitations readers should be aware of. Firstly, the reason the antibiotic was prescribed is not known, therefore no judgement on the appropriateness or otherwise can be drawn.
Furthermore, this collection does not capture antibiotics:
- prescribed but not dispensed
- provided over-the-counter, such as trimethoprim (prescribed trimethoprim is included)
- dispensed on a practitioner supply order (PSO).
PSO is used where an individual prescription may not be possible, for example, in the school-based rheumatic fever prevention programme. On average, PSO represents around 4 percent of antibiotics dispensed, but the use of PSO ranges between district health boards (DHBs) from 0.9 percent to 19 percent.
- Since 2015, there has been a 6 percent reduction in the rate of antibiotic dispensing in Aotearoa New Zealand. This equates to 133,000 fewer people dispensed an antibiotic in 2018 than in 2015.
- Of people who visited their GP in 2018, around half were dispensed at least one systemic antibiotic. The prescription could have come from any prescriber.
- North Island DHBs dispensed statistically significantly more systemic antibiotics to 0–4-year-olds than South Island DHBs.
- Antibiotic use was highest in 2018 in the youngest and oldest in the population:
- Of 0–4-year-olds, 63 percent were dispensed an antibiotic.
- Of those aged 85 years and over living in aged residential care (ARC), 66 percent received an antibiotic.
- Topical antibiotic use has reduced over the last three years, from 6 percent to less than 4 percent. This varied two-fold between DHBs.
- Some antibiotics were dispensed 26 percent more in winter than in summer. Increased dispensing in winter may indicate that antibiotics are being dispensed for individuals with colds and flus, and therefore may represent an opportunity to reduce antibiotic use.
- Eighty-one percent of penicillins dispensed are broad-spectrum.
- The dispensing of antibiotics specifically indicated for urinary tract infections (UTIs) increased sharply with age and for people living in ARC. Since 2015 there has been a 7 percent reduction in the dispensing of antibiotics for UTIs in ARC.
- On average, about one-third of people were dispensed an antibiotic within 30 days of a medical and surgical admission.
Of people who visited their GP in a year, around half were dispensed at least one systemic antibiotic
- The highest rate of antibiotic dispensing was in 0–4-year-olds, with 63 percent dispensed in 2018, followed by 57 percent of 5–9-year-olds and 51 percent of 15–24 years old. Antibiotic use increased again with older age.
- DHB variation was 1.4-fold, with wider variation between DHBs in ethnic rates.
- Pacific peoples had the highest dispensing rate by ethnic group, particularly in young children, with 80 percent of 0–4-year-olds dispensed an antibiotic in 2018. This has reduced significantly since 2015, when 88 percent of young Pacific children were dispensed an antibiotic. Māori also had a high rate of antibiotic use, predominantly in the 0–9-year age groups. It is not known whether this use is appropriate or not.
- These observations should be interpreted in the context of research showing higher rates of infectious disease in Māori and Pacific peoples[4].
- The relative rate of antibiotic use in 15–24-year-olds, particularly in those of European/Other ethnicity (52 percent) raises questions about which antibiotics are being prescribed the most and for what indication.
When all dispensings in a year were calculated against all GP visits, the rate of dispensing was lower, at 36 percent. This lower rate reflects that, while around half the people who attended primary care in a year were dispensed an antibiotic, this does not mean that half of GP visits resulted in an antibiotic being dispensed.
- During 2018, 1.8 million people were dispensed a systemic antibiotic in the community and they received a total of 3.65 million antibiotic dispensings. Since 2015, this represents 381,000 fewer antibiotic dispensings.
- To provide context, in 2018, antibiotics were dispensed to 183,000 people in the month following hospital admission, most of which were medical admissions (134,000). Overall, the majority of community prescriptions originated from primary care.
- For a further analysis reporting which antibiotics were dispensed by age group, see the paper by Whyler et al[5].
People living in ARC were dispensed more antibiotics than those living in the community
- In those aged 85 years and over living in ARC, DHB rates ranged from 52 percent to 75 percent, with an average rate of 66 percent. In 2018, Pacific peoples aged 85 years and over living in ARC were dispensed 25 percent more antibiotics than in 2017. This compares with a national average of 55 percent for people 85 years and over living in the community.
- Eight North Island DHBs had statistically significantly higher dispensing rates in 2018. The highest in the last four years.
Topical antibiotic use has decreased statistically significantly since 2015, from 6 percent to 3.5 percent
- Use varied two-fold between DHBs.
- As with systemic antibiotics, rates were higher in young children and in Māori and Pacific populations. However, previous research has shown that the rate of hospital admission for skin and soft tissue infection are highest in Māori and Pacific peoples[6].The reduction in rates may reflect more appropriate use of topical antibiotics.
Systemic antibiotics were prescribed 26 percent more in winter than in summer
- Since 2015, antibiotic dispensing over the winter months has decreased from 39 percent to 26 percent higher than in summer. However this apparent improvement should be interpreted with caution because seasonal variation tends to vary between years.
- Seasonal antibiotic use was highest in the young, with 54 percent more antibiotics dispensed in winter in those aged 0–4 years, compared with an increase of around 14 percent in those aged 75 years and over.
- Increased prescribing in winter may indicate that antibiotics are being prescribed for viral respiratory infections. A study in the United Kingdom found social norm feedback reduced the seasonal increase in antibiotic prescribing over winter[7]. This may represent an opportunity to reduce antibiotic use. bpacnz has guidance on managing the cold season without antibiotics[8].
- Typically, seasonal variation was greatest in the North Island DHBs. This may reflect differences in population groups or other factors such as climate.
Of those dispensed a penicillin, 81 percent of people received a broad-spectrum penicillin
- This Atlas domain uses the New Zealand Formulary classification, which defines amoxicillin and amoxicillin with clavulanic acid as broad-spectrum. All other penicillins are narrow-spectrum.
- As a percentage of all penicillin dispensed, amoxicillin and amoxicillin with clavulanic acid was used 81 percent of the time for all age groups. Broad-spectrum penicillin use was highest in those aged 0–4 years at 93 percent; this equated to around 131,000 children in 2018. This raises questions such as:
- which conditions in box 1 are likely to be relevant for 0–4-year-olds
- are there other factors involved, for example, formulation and palatability?
- Broad-spectrum antibiotics are associated with antimicrobial resistance and are generally recommended only for specific indications[8]. According to the New Zealand Formulary, amoxicillin is used principally in the treatment of community-acquired pneumonia, sinusitis and middle-ear infections. Amoxicillin with clavulanic acid is used in severe or complicated infections[9].
|
Box 1 Conditions where amoxicillin is the first-choice antibiotic:
|
|
Conditions where amoxicillin with clavulanic acid is the first-choice antibiotic:
|
|
Source: bpacnz. 2017. Antibiotics: choices for common infections. URL: https://bpac.org.nz/antibiotics/guide.aspx Note: this list contains common conditions only and is not a complete list. |
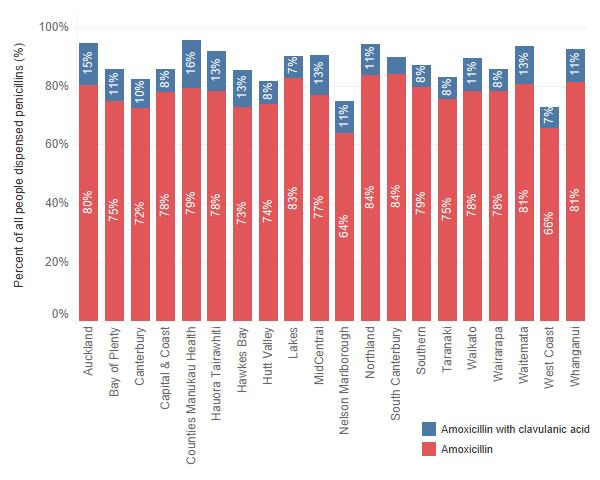
Figure 1: Amoxicillin and amoxicillin with clavulanic acid as a percentage of all penicillins for those aged 0–4 years in 2018, by DHB
Eleven percent of people (~434,000) who visited their GP were dispensed amoxicillin with clavulanic acid at least once in 2018
- Amoxicillin with clavulanic acid is a broad-spectrum antibiotic. Overall, dispensing rates increased with age.
- Use has reduced from 14 percent to 11 percent of people since 2015, a reduction of 87,000 people. This reduction is highest among 0–4-year-olds, from 16 percent in 2015 to 10 percent in 2018. This equates to 18,000 people.
- Pacific peoples were dispensed statistically significantly more amoxicillin with clavulanic acid than any other ethnic group in 2018.
- Amoxicillin with clavulanic acid dispensing rates were higher in the North Island.
- DHB variation is greater than two-fold, ranging from 7 percent to 16 percent.
The dispensing of antibiotics specifically indicated for UTIs increased sharply with age and for people living in ARC
- The rate of antibiotics dispensed for a UTI varied according to where people lived: for those aged 65–74 years, 6 percent of people in the community received an antibiotic for a UTI compared with 18 percent of those living in ARC.
- In the community, dispensing was four times higher in women than in men and was statistically significantly higher in those of European/Other ethnicity. Female ARC residents received 1.7 times more antibiotics for UTIs than male ARC residents.
- In people aged 65 years and over, asymptomatic bacteriuria and UTIs can be common[10]. A Cochrane Review (2015) concluded there was no clinical benefit from treating asymptomatic bacteriuria[10].
On average, about one-third of people were dispensed an antibiotic within 30 days of a medical and surgical admission
- This equates to antibiotics dispensed to 134,000 people following a medical discharge and 49,000 people following a surgical discharge.
- The Atlas domain presenting data about antibiotic dispensing following major surgery shows a similar result, where 32 percent of people were dispensed an antibiotic.
- There was 1.3-fold variation between DHBs for both admission types.
- It is recommended that, along with good infection prevention and control, antibiotics be prescribed only when needed, with the narrowest spectrum of antimicrobial activity. To prevent surgical site infection, NICE recommends antibiotics be used only in cases where there is an increased risk of infection; well-designed studies have shown a benefit of surgical antimicrobial prophylaxis[11]. Previous research in Aotearoa New Zealand concluded a significant proportion of antibiotics prescribed to patients discharged following surgery was inappropriate and recommended enhanced antimicrobial stewardship in this area[12]. It appears there may be a similar pattern in medical patients.
- Figure 2 shows that 50 percent of medical discharges and 43 percent of surgical discharges had their antibiotic dispensed on the same or next day of discharge.
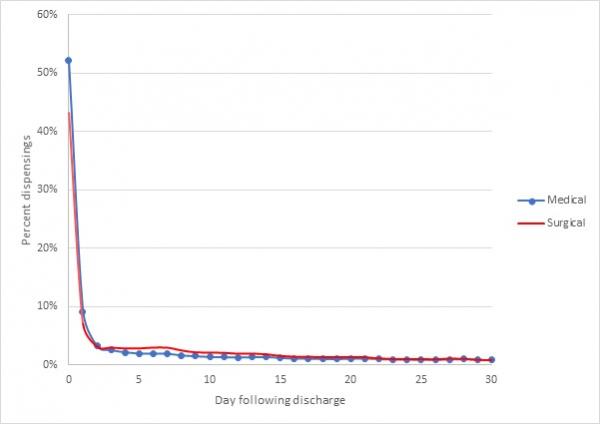
Figure 2: Timing of antibiotic on discharge, by admission type in 2018
Data for this Atlas domain was drawn from the Pharmaceutical Collection, which contains claim and payment information from community pharmacies for subsidised dispensing. This collection does not allow for analysis of patients’ condition or the effectiveness of dose provided. This means it was not possible to assess the appropriateness or otherwise of prescribing. Unsubsidised dispensing is not included in this analysis; nor does it indicate if people took the medicine. As noted above, antibiotics dispensed under a PSO are not included. This means that children receiving an antibiotic through a school sore throat programme will not be included in the data.
The methodology is provided here (290KB, pdf).
Note: this analysis focuses on the number of people dispensed an antibiotic, not on the number of prescriptions (except for indicator 1b). We have used this approach because interpreting what the number of dispensed prescriptions might mean is complicated by differences in prescribing frequency, formulation and dose. We recommend further local analysis of people receiving antibiotics that accounts for these factors and the person’s clinical condition.
- Why do rates vary between DHBs? How much can be explained by differences in patient population?
- Does the pattern of prescribing seem appropriate? Is it consistent with guidelines and are these regularly reviewed?
- What is the impact of crowding on rates of antimicrobial dispensing? Do DHBs with a higher prevalence of overcrowded housing also have higher incidence of infectious disease?
- Baker MG, Barnard LT, Kvalsvig A, et al. 2012. Increasing incidence of serious infectious diseases and inequalities in New Zealand: a national epidemiological study. Lancet 379(9821): 1112–9.
- Hallsworth M, Chadborn T, Sallis A, et al. 2016. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet 387(10029): 1743–52.
- Ministry of Health and Ministry for Primary Industries. 2017. New Zealand Antimicrobial Resistance Action Plan. Wellington: Ministry of Health. URL: https://www.health.govt.nz/publications/new-zealand-antimicrobial-resistance-action-plan#mig (accessed 19 March 2019).
- Norris P, Horsburgh S, Keown S, et al. 2011. Too much and too little? Prevalence and extent of antibiotic use in a New Zealand region. J Antimicrob Chemother 66(8): 1921–6.
- The Royal New Zealand College of General Practitioners. 2015. Antibiotics and antimicrobial resistance: avoiding a post-antibiotic era. May 2015. Policy Brief. Issue 3. URL: https://www.rnzcgp.org.nz/gpdocs/New-website/Advocacy/05.2015-Antibiotics-and-antimicrobial-resistance-Policy-brief-1.pdf (accessed March 2019).
- Whyler N, Tomlin A, Tilyard M, et al. 2018 Ethnic disparities in community antibacterial dispensing in New Zealand, 2015. NZ Med J 131(1480): 50–60.
- Williamson DA, Roos RF, Verrall A. 2016. Antibiotic consumption in New Zealand, 2006–2014. Porirua: The Institute of Environmental Science and Research Ltd. URL: https://www.esr.cri.nz/media/nrxfs5db/antibiotic_consumption_report_final.pdf(accessed March 2019).
- EPIC Antibiotics: Three data stories describing use of antibiotic medicines in New Zealand, published by He Ako Hiringa. Data current to last three months. Updated quarterly. Practitioner, practice, and national data: https://epic.akohiringa.co.nz/antibiotics
- Kotahitanga - Uniting Aotearoa against infectious disease and antimicrobial resistance. A report from the Prime Minister’s Chief Science Advisor. 2021:
https://www.pmcsa.ac.nz/files/2020/01/Short-report-web-v4.pdf
Resources from bpacnz:
- Antibiotics guide: https://bpac.org.nz/antibiotics/guide.aspx
- Topical antibiotics: https://bpac.org.nz/2018/topical-antibiotics.aspx
- Cold season: https://bpac.org.nz/2018/cold-season.aspx
- Duffy E, Ritchie S, Metcalfe S, et al. 2018. Antibacterials dispensed in the community comprise 85%-95% of total human antibacterial consumption. J Clin Pharm Ther 43(1): 59–64.
- Metcalfe S, Bhawan S, Vallabh M, et al. 2019. Over and under? Ethnic inequities in community antibacterial prescribing. NZMJ 132(1488).
- Wennberg JE. 2011. Time to tackle unwarranted variations in practice. BMJ 342: d1513.
- Baker MG, Barnard LT, Kvalsvig A, et al. 2012. Increasing incidence of serious infectious diseases and inequalities in New Zealand: a national epidemiological study. Lancet 379(9821): 1112–9.
- Whyler N, Tomlin A, Tilyard M, et al. 2018. Ethnic disparities in community antibacterial dispensing in New Zealand, 2015. NZ Med J 131(1480): 50–60.
- Williamson DA, Zhang J, Ritchie SR, et al. 2014. Staphylococcus aureus infections in New Zealand, 2000-2011. Emerg Infect Dis 20(7): 1156–61.
- Hallsworth M, Chadborn T, Sallis A, et al. 2016. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet 387(10029): 1743–52.
- bpacnz. 2018. Cold season: managing without antibiotics. URL: www.bpac.org.nz/2018/cold-season.aspx (accessed November 2018).
- New Zealand Formulary (NZF). 2019. NZF v81. URL: www.nzf.org.nz (accessed March 2019).
- Nicolle LE. 2016. Urinary tract infections in the older adult. Clin Geriatr Med 32: 523–38.
- Zalmanovici Trestioreanu A, Lador A, et al. Antibiotics for asymptomatic bacteriuria. Cochrane Database Syst Rev 2015, Issue 4, Art. No. CD009534. URL: www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009534.pub2/epdf/full (accessed November 2018).
- NICE. Surgical site infection – prevention and treatment. London: NICE. URL: https://pathways.nice.org.uk/pathways/prevention-and-control-of-healthcare-associated-infections (accessed December 2018).
- De Almeida M, Gerard C, Freeman JT, et al. 2018. Inappropriate prescribing of antibiotics following discharge after major surgery: an area for improvement. NZ Med J 131(1475): 35–43.
Background
Antimicrobial resistance is emerging as a problem worldwide. Overuse of antibiotics is one of the causes of antimicrobial resistance.
In Aotearoa New Zealand, up to 95 percent of antibiotics are dispensed in the community[1], suggesting that a focus on promoting appropriate community use is an important way to address antimicrobial resistance. Recent analysis indicates there is likely to be a mix of underuse and overuse of antibacterials relative to health need, particularly in Māori and Pacific peoples. This suggests the balance of under- and over-prescribing of antibiotics in Aotearoa New Zealand needs to be better understood[2].
The purpose of this Atlas of Healthcare Variation domain is to highlight regional and demographic variation in community antibiotic use, with the goal of prompting debate and raising questions about why differences exist. Professor John Wennberg defined unwarranted variation as being variation that cannot be explained by differences in either patient need or patient preference. This Atlas domain invites readers to ask questions to understand how much antibiotic prescribing can be attributed to patient need and how much might reflect unwarranted variation[3].
Data comes from the Pharmaceutical Collection and there are limitations readers should be aware of. Firstly, the reason the antibiotic was prescribed is not known, therefore no judgement on the appropriateness or otherwise can be drawn.
Furthermore, this collection does not capture antibiotics:
- prescribed but not dispensed
- provided over-the-counter, such as trimethoprim (prescribed trimethoprim is included)
- dispensed on a practitioner supply order (PSO).
PSO is used where an individual prescription may not be possible, for example, in the school-based rheumatic fever prevention programme. On average, PSO represents around 4 percent of antibiotics dispensed, but the use of PSO ranges between district health boards (DHBs) from 0.9 percent to 19 percent.
Key observations
- Since 2015, there has been a 6 percent reduction in the rate of antibiotic dispensing in Aotearoa New Zealand. This equates to 133,000 fewer people dispensed an antibiotic in 2018 than in 2015.
- Of people who visited their GP in 2018, around half were dispensed at least one systemic antibiotic. The prescription could have come from any prescriber.
- North Island DHBs dispensed statistically significantly more systemic antibiotics to 0–4-year-olds than South Island DHBs.
- Antibiotic use was highest in 2018 in the youngest and oldest in the population:
- Of 0–4-year-olds, 63 percent were dispensed an antibiotic.
- Of those aged 85 years and over living in aged residential care (ARC), 66 percent received an antibiotic.
- Topical antibiotic use has reduced over the last three years, from 6 percent to less than 4 percent. This varied two-fold between DHBs.
- Some antibiotics were dispensed 26 percent more in winter than in summer. Increased dispensing in winter may indicate that antibiotics are being dispensed for individuals with colds and flus, and therefore may represent an opportunity to reduce antibiotic use.
- Eighty-one percent of penicillins dispensed are broad-spectrum.
- The dispensing of antibiotics specifically indicated for urinary tract infections (UTIs) increased sharply with age and for people living in ARC. Since 2015 there has been a 7 percent reduction in the dispensing of antibiotics for UTIs in ARC.
- On average, about one-third of people were dispensed an antibiotic within 30 days of a medical and surgical admission.
Of people who visited their GP in a year, around half were dispensed at least one systemic antibiotic
- The highest rate of antibiotic dispensing was in 0–4-year-olds, with 63 percent dispensed in 2018, followed by 57 percent of 5–9-year-olds and 51 percent of 15–24 years old. Antibiotic use increased again with older age.
- DHB variation was 1.4-fold, with wider variation between DHBs in ethnic rates.
- Pacific peoples had the highest dispensing rate by ethnic group, particularly in young children, with 80 percent of 0–4-year-olds dispensed an antibiotic in 2018. This has reduced significantly since 2015, when 88 percent of young Pacific children were dispensed an antibiotic. Māori also had a high rate of antibiotic use, predominantly in the 0–9-year age groups. It is not known whether this use is appropriate or not.
- These observations should be interpreted in the context of research showing higher rates of infectious disease in Māori and Pacific peoples[4].
- The relative rate of antibiotic use in 15–24-year-olds, particularly in those of European/Other ethnicity (52 percent) raises questions about which antibiotics are being prescribed the most and for what indication.
When all dispensings in a year were calculated against all GP visits, the rate of dispensing was lower, at 36 percent. This lower rate reflects that, while around half the people who attended primary care in a year were dispensed an antibiotic, this does not mean that half of GP visits resulted in an antibiotic being dispensed.
- During 2018, 1.8 million people were dispensed a systemic antibiotic in the community and they received a total of 3.65 million antibiotic dispensings. Since 2015, this represents 381,000 fewer antibiotic dispensings.
- To provide context, in 2018, antibiotics were dispensed to 183,000 people in the month following hospital admission, most of which were medical admissions (134,000). Overall, the majority of community prescriptions originated from primary care.
- For a further analysis reporting which antibiotics were dispensed by age group, see the paper by Whyler et al[5].
People living in ARC were dispensed more antibiotics than those living in the community
- In those aged 85 years and over living in ARC, DHB rates ranged from 52 percent to 75 percent, with an average rate of 66 percent. In 2018, Pacific peoples aged 85 years and over living in ARC were dispensed 25 percent more antibiotics than in 2017. This compares with a national average of 55 percent for people 85 years and over living in the community.
- Eight North Island DHBs had statistically significantly higher dispensing rates in 2018. The highest in the last four years.
Topical antibiotic use has decreased statistically significantly since 2015, from 6 percent to 3.5 percent
- Use varied two-fold between DHBs.
- As with systemic antibiotics, rates were higher in young children and in Māori and Pacific populations. However, previous research has shown that the rate of hospital admission for skin and soft tissue infection are highest in Māori and Pacific peoples[6].The reduction in rates may reflect more appropriate use of topical antibiotics.
Systemic antibiotics were prescribed 26 percent more in winter than in summer
- Since 2015, antibiotic dispensing over the winter months has decreased from 39 percent to 26 percent higher than in summer. However this apparent improvement should be interpreted with caution because seasonal variation tends to vary between years.
- Seasonal antibiotic use was highest in the young, with 54 percent more antibiotics dispensed in winter in those aged 0–4 years, compared with an increase of around 14 percent in those aged 75 years and over.
- Increased prescribing in winter may indicate that antibiotics are being prescribed for viral respiratory infections. A study in the United Kingdom found social norm feedback reduced the seasonal increase in antibiotic prescribing over winter[7]. This may represent an opportunity to reduce antibiotic use. bpacnz has guidance on managing the cold season without antibiotics[8].
- Typically, seasonal variation was greatest in the North Island DHBs. This may reflect differences in population groups or other factors such as climate.
Of those dispensed a penicillin, 81 percent of people received a broad-spectrum penicillin
- This Atlas domain uses the New Zealand Formulary classification, which defines amoxicillin and amoxicillin with clavulanic acid as broad-spectrum. All other penicillins are narrow-spectrum.
- As a percentage of all penicillin dispensed, amoxicillin and amoxicillin with clavulanic acid was used 81 percent of the time for all age groups. Broad-spectrum penicillin use was highest in those aged 0–4 years at 93 percent; this equated to around 131,000 children in 2018. This raises questions such as:
- which conditions in box 1 are likely to be relevant for 0–4-year-olds
- are there other factors involved, for example, formulation and palatability?
- Broad-spectrum antibiotics are associated with antimicrobial resistance and are generally recommended only for specific indications[8]. According to the New Zealand Formulary, amoxicillin is used principally in the treatment of community-acquired pneumonia, sinusitis and middle-ear infections. Amoxicillin with clavulanic acid is used in severe or complicated infections[9].
|
Box 1 Conditions where amoxicillin is the first-choice antibiotic:
|
|
Conditions where amoxicillin with clavulanic acid is the first-choice antibiotic:
|
|
Source: bpacnz. 2017. Antibiotics: choices for common infections. URL: https://bpac.org.nz/antibiotics/guide.aspx Note: this list contains common conditions only and is not a complete list. |

Figure 1: Amoxicillin and amoxicillin with clavulanic acid as a percentage of all penicillins for those aged 0–4 years in 2018, by DHB
Eleven percent of people (~434,000) who visited their GP were dispensed amoxicillin with clavulanic acid at least once in 2018
- Amoxicillin with clavulanic acid is a broad-spectrum antibiotic. Overall, dispensing rates increased with age.
- Use has reduced from 14 percent to 11 percent of people since 2015, a reduction of 87,000 people. This reduction is highest among 0–4-year-olds, from 16 percent in 2015 to 10 percent in 2018. This equates to 18,000 people.
- Pacific peoples were dispensed statistically significantly more amoxicillin with clavulanic acid than any other ethnic group in 2018.
- Amoxicillin with clavulanic acid dispensing rates were higher in the North Island.
- DHB variation is greater than two-fold, ranging from 7 percent to 16 percent.
The dispensing of antibiotics specifically indicated for UTIs increased sharply with age and for people living in ARC
- The rate of antibiotics dispensed for a UTI varied according to where people lived: for those aged 65–74 years, 6 percent of people in the community received an antibiotic for a UTI compared with 18 percent of those living in ARC.
- In the community, dispensing was four times higher in women than in men and was statistically significantly higher in those of European/Other ethnicity. Female ARC residents received 1.7 times more antibiotics for UTIs than male ARC residents.
- In people aged 65 years and over, asymptomatic bacteriuria and UTIs can be common[10]. A Cochrane Review (2015) concluded there was no clinical benefit from treating asymptomatic bacteriuria[10].
On average, about one-third of people were dispensed an antibiotic within 30 days of a medical and surgical admission
- This equates to antibiotics dispensed to 134,000 people following a medical discharge and 49,000 people following a surgical discharge.
- The Atlas domain presenting data about antibiotic dispensing following major surgery shows a similar result, where 32 percent of people were dispensed an antibiotic.
- There was 1.3-fold variation between DHBs for both admission types.
- It is recommended that, along with good infection prevention and control, antibiotics be prescribed only when needed, with the narrowest spectrum of antimicrobial activity. To prevent surgical site infection, NICE recommends antibiotics be used only in cases where there is an increased risk of infection; well-designed studies have shown a benefit of surgical antimicrobial prophylaxis[11]. Previous research in Aotearoa New Zealand concluded a significant proportion of antibiotics prescribed to patients discharged following surgery was inappropriate and recommended enhanced antimicrobial stewardship in this area[12]. It appears there may be a similar pattern in medical patients.
- Figure 2 shows that 50 percent of medical discharges and 43 percent of surgical discharges had their antibiotic dispensed on the same or next day of discharge.

Figure 2: Timing of antibiotic on discharge, by admission type in 2018
Data sources and limitations
Data for this Atlas domain was drawn from the Pharmaceutical Collection, which contains claim and payment information from community pharmacies for subsidised dispensing. This collection does not allow for analysis of patients’ condition or the effectiveness of dose provided. This means it was not possible to assess the appropriateness or otherwise of prescribing. Unsubsidised dispensing is not included in this analysis; nor does it indicate if people took the medicine. As noted above, antibiotics dispensed under a PSO are not included. This means that children receiving an antibiotic through a school sore throat programme will not be included in the data.
The methodology is provided here (290KB, pdf).
Note: this analysis focuses on the number of people dispensed an antibiotic, not on the number of prescriptions (except for indicator 1b). We have used this approach because interpreting what the number of dispensed prescriptions might mean is complicated by differences in prescribing frequency, formulation and dose. We recommend further local analysis of people receiving antibiotics that accounts for these factors and the person’s clinical condition.
Questions this Atlas domain may prompt
- Why do rates vary between DHBs? How much can be explained by differences in patient population?
- Does the pattern of prescribing seem appropriate? Is it consistent with guidelines and are these regularly reviewed?
- What is the impact of crowding on rates of antimicrobial dispensing? Do DHBs with a higher prevalence of overcrowded housing also have higher incidence of infectious disease?
Recommended reading
- Baker MG, Barnard LT, Kvalsvig A, et al. 2012. Increasing incidence of serious infectious diseases and inequalities in New Zealand: a national epidemiological study. Lancet 379(9821): 1112–9.
- Hallsworth M, Chadborn T, Sallis A, et al. 2016. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet 387(10029): 1743–52.
- Ministry of Health and Ministry for Primary Industries. 2017. New Zealand Antimicrobial Resistance Action Plan. Wellington: Ministry of Health. URL: https://www.health.govt.nz/publications/new-zealand-antimicrobial-resistance-action-plan#mig (accessed 19 March 2019).
- Norris P, Horsburgh S, Keown S, et al. 2011. Too much and too little? Prevalence and extent of antibiotic use in a New Zealand region. J Antimicrob Chemother 66(8): 1921–6.
- The Royal New Zealand College of General Practitioners. 2015. Antibiotics and antimicrobial resistance: avoiding a post-antibiotic era. May 2015. Policy Brief. Issue 3. URL: https://www.rnzcgp.org.nz/gpdocs/New-website/Advocacy/05.2015-Antibiotics-and-antimicrobial-resistance-Policy-brief-1.pdf (accessed March 2019).
- Whyler N, Tomlin A, Tilyard M, et al. 2018 Ethnic disparities in community antibacterial dispensing in New Zealand, 2015. NZ Med J 131(1480): 50–60.
- Williamson DA, Roos RF, Verrall A. 2016. Antibiotic consumption in New Zealand, 2006–2014. Porirua: The Institute of Environmental Science and Research Ltd. URL: https://www.esr.cri.nz/media/nrxfs5db/antibiotic_consumption_report_final.pdf(accessed March 2019).
Resources
- EPIC Antibiotics: Three data stories describing use of antibiotic medicines in New Zealand, published by He Ako Hiringa. Data current to last three months. Updated quarterly. Practitioner, practice, and national data: https://epic.akohiringa.co.nz/antibiotics
- Kotahitanga - Uniting Aotearoa against infectious disease and antimicrobial resistance. A report from the Prime Minister’s Chief Science Advisor. 2021:
https://www.pmcsa.ac.nz/files/2020/01/Short-report-web-v4.pdf
Resources from bpacnz:
- Antibiotics guide: https://bpac.org.nz/antibiotics/guide.aspx
- Topical antibiotics: https://bpac.org.nz/2018/topical-antibiotics.aspx
- Cold season: https://bpac.org.nz/2018/cold-season.aspx
References
- Duffy E, Ritchie S, Metcalfe S, et al. 2018. Antibacterials dispensed in the community comprise 85%-95% of total human antibacterial consumption. J Clin Pharm Ther 43(1): 59–64.
- Metcalfe S, Bhawan S, Vallabh M, et al. 2019. Over and under? Ethnic inequities in community antibacterial prescribing. NZMJ 132(1488).
- Wennberg JE. 2011. Time to tackle unwarranted variations in practice. BMJ 342: d1513.
- Baker MG, Barnard LT, Kvalsvig A, et al. 2012. Increasing incidence of serious infectious diseases and inequalities in New Zealand: a national epidemiological study. Lancet 379(9821): 1112–9.
- Whyler N, Tomlin A, Tilyard M, et al. 2018. Ethnic disparities in community antibacterial dispensing in New Zealand, 2015. NZ Med J 131(1480): 50–60.
- Williamson DA, Zhang J, Ritchie SR, et al. 2014. Staphylococcus aureus infections in New Zealand, 2000-2011. Emerg Infect Dis 20(7): 1156–61.
- Hallsworth M, Chadborn T, Sallis A, et al. 2016. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet 387(10029): 1743–52.
- bpacnz. 2018. Cold season: managing without antibiotics. URL: www.bpac.org.nz/2018/cold-season.aspx (accessed November 2018).
- New Zealand Formulary (NZF). 2019. NZF v81. URL: www.nzf.org.nz (accessed March 2019).
- Nicolle LE. 2016. Urinary tract infections in the older adult. Clin Geriatr Med 32: 523–38.
- Zalmanovici Trestioreanu A, Lador A, et al. Antibiotics for asymptomatic bacteriuria. Cochrane Database Syst Rev 2015, Issue 4, Art. No. CD009534. URL: www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009534.pub2/epdf/full (accessed November 2018).
- NICE. Surgical site infection – prevention and treatment. London: NICE. URL: https://pathways.nice.org.uk/pathways/prevention-and-control-of-healthcare-associated-infections (accessed December 2018).
- De Almeida M, Gerard C, Freeman JT, et al. 2018. Inappropriate prescribing of antibiotics following discharge after major surgery: an area for improvement. NZ Med J 131(1475): 35–43.
